Bio-Pharma Operations & GMP Engineering Partner for Singapore & Southeast Asia
VentriTech Solutions helps bio-pharmaceutical manufacturers reduce unplanned downtime, de-risk audits, and cut OPEX through automation, EPF projects, and outsourced operations support.
Reduce unplanned equipment downtime across critical utilities and process equipment
Deploy cGMP-trained personnel for specialised cleaning, sensitive equipment handling & GMP logistics
Scale engineering capacity without increasing permanent headcount
Strengthen data integrity & audit readiness for FDA, EMA, and HSA inspections
Bespoke Engineering for Unmet Needs in GMP Manufacturing
Growing volume of equipment- and automation-related deviations, CAPAs and audit observations linked to data integrity and documentation gaps
Skilled operators and engineers tied up in non-core but GMP-critical tasks such as specialised cleaning, re-labelling, repacking and GMP logistics
Chronic unplanned downtime and slow troubleshooting on critical process equipment and utilities
Key Operational & Engineering Challenges
How VentriTech Supports Your Operations
VentriTech combines on-site engineering specialists, cGMP-trained outsourced operations teams and structured OE / Lean Six Sigma methods to stabilise critical equipment, reduce deviations and audit findings, and take GMP-critical but non-core work off your internal teams. We help you cut unplanned downtime, clear engineering backlogs and free your best people to focus on product, patients and strategic improvement.
We extend this support with vtrac – our AI-enabled troubleshooting and GxP documentation assistant built specifically for bio-pharmaceutical manufacturing. vtrac helps shopfloor technicians and engineers diagnose equipment issues faster, pre-draft deviation, change control and incident reports, and run gap analyses on SOPs and WIs against global regulatory expectations, so every shift is more compliant, consistent and productive.
Ventritech Solutions partners with Heads of Engineering, Manufacturing, and QA in GMP-regulated bio-pharmaceutical facilities. We step in when internal teams are stretched, closing gaps in automation, project engineering, and day-to-day technical support so your lines stay compliant, reliable, and on-schedule.
Challenges in cGMP manufacturing
How we help
Targeted Solutions for Critical Operations
In high-stakes bio-pharma manufacturing, tasks quickly become a bottleneck. Digital transformations, while crucial, often involve routine operations, technical support, projects, and automation enhancements that distract your top talent from focusing on products and patients. Ventritech absorbs this load so your teams stay focused while we keep your manufacturing foundation compliant, reliable, and audit-ready.
Operational Excellence & Engineering Consultancy
Use decades of pharma experience and TPS / Lean methods to troubleshoot chronic issues, improve line performance, and build a practical OPEX-reduction roadmap.
Staff Augmentation & Operations Support
Scale your team without permanent headcount. Deploy cGMP-trained staff for shutdowns, specialised cleaning, sensitive equipment handling, projects, re-labelling, re-packing and other GMP logistics.


Digital Transformation & Automation
Modernise operations with real-time plant data, DeltaV / PLC / SCADA upgrades, and secure integrations that support 21 CFR Part 11 & ALCOA+.

EPF (Engineering, Procurement, Fabrication)
Deliver greenfield and brownfield projects, capacity expansions, and utility upgrades from concept to commissioning with GMP-aligned design, procurement & fabrication.

AI FOR PHARMA OPERATIONS
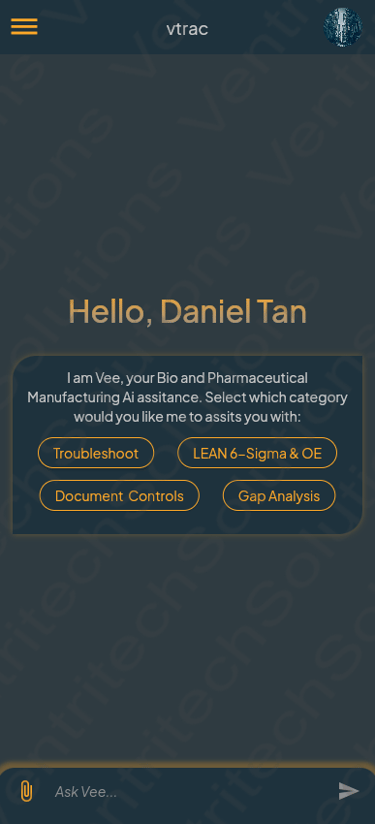
Meet vtrac: Your AI Co-Pilot for Pharma Engineering & Operations
Built by VentriTech specifically for cGMP facilities, vtrac sits alongside your teams to speed up troubleshooting and documentation — not replace their judgement.
Guided troubleshooting for critical equipment based on your asset data, deviations and OE methods
AI-assisted drafting of deviations, change controls, incident reports and CAPA outlines aligned with GxP expectations
Gap analysis support for SOPs and WIs against global regulatory standards to keep documents inspection-ready
Designed for shopfloor and engineering teams — fast, focused and usable from any device


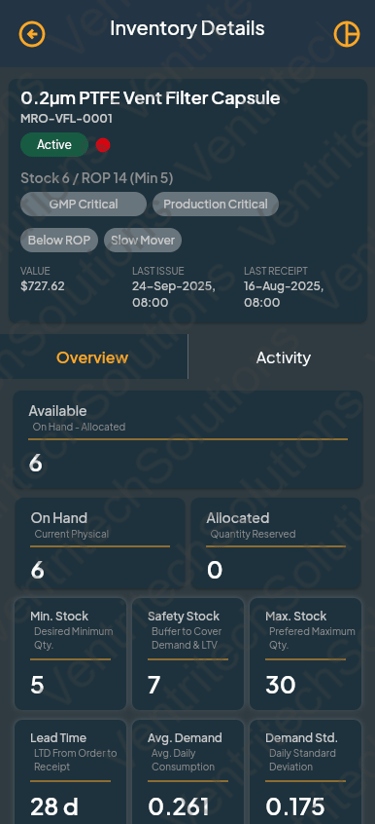
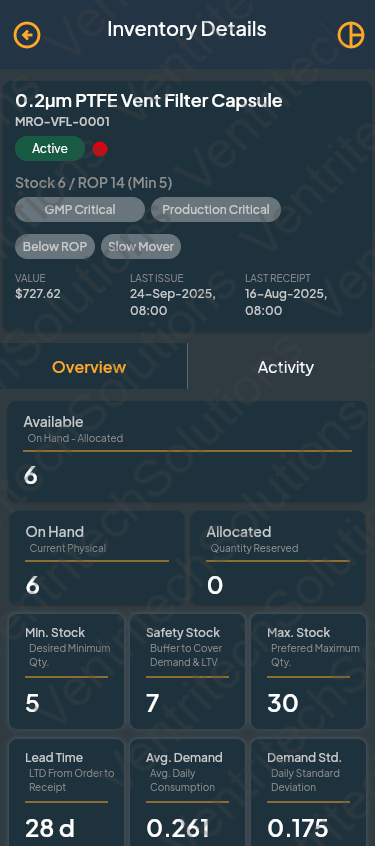
Begin Your Operational Excellence Journey
Share a few details about your facility, and our technical leadership will review your situation and respond within 3-5 business days.
We can schedule a 30-minute operations gap assessment or an executive briefing tailored to your site.
Transform Operational Gaps into Competitive Advantages
All rights reserved.
© Ventritech Solutions Pte. Ltd. 2025 Registered in Singapore No. 202216713Z Registered Office: 51 Goldhill Plaza, #07-07, Singapore 308900
